Basics|Precision pumping technology 7-2. Vaporization of Liquid (Evaporation)
Vaporization of Liquid (Evaporation)
The example using beer in "7-1. Cavitation" demonstrated that carbon dioxide gas dissolved in beer diffuses out of the liquid when there is a decrease in pressure.
In this section, let's look at cases where the liquid itself turns into gas (vaporization) instead of dissolved gas escaping from the liquid.
Beer is a mixture of water, alcohol, and carbon dioxide gas, but to make the explanation simple, we will use pure water.
Water boils at 100 degrees Centigrade. This is supposedly common knowledge, but is it actually true?
In fact, water only boils at 100°C when the ambient pressure is 1 atmosphere (= 0.1013 MPa).
Water molecules start moving more intensely in elevated temperatures. Water molecules are bonded to each other while the temperature is low, but when the temperature rises to a certain point, the bond breaks due to the increased molecular motion.
Water boiling means that the molecules that broke free from the bond (water vapor) surmount the force on the liquid surface and jump out. This temperature is called the boiling point.
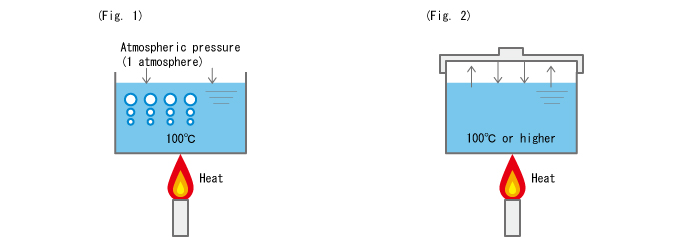
In the case of an open container (not airtight/watertight) as shown in Fig. 1, the force on the liquid surface is the air pressure (atmospheric pressure).
Here, the temperature at which water begins boiling, surmounting the atmospheric pressure (1 atmosphere) is 100°C. Under this condition, once water starts boiling, the temperature remains at 100°C until water is completely gone.
Next, we cover the container, sealing it completely as shown in Fig. 2.
As we heat water in this state, unlike with an open container, the temperature keeps rising and finally goes over 100°C. In a completely sealed container, the internal gas pressure increases and presses down on the liquid surface--this prevents the water inside from boiling even when the temperature reaches 100°C.
Specifically, water boils at about 100°C under atmospheric pressure (0.1 MPa), at about 120°C under 0.2 MPa, and at around 140°C under 0.37 MPa.
The pressure cooker uses this principle.
A pressure cooker can cook rice at temperatures over 100°C by creating a high-pressure (within atmospheric pressure + 0.1 MPa) environment inside the cooker container. As a result, rice can be cooked to perfection in a short time.
Let's now consider the other way around.
Turning around the example of the pressure cooker, we will now lower the pressure inside the sealed container. We do this by removing the air inside the container using a vacuum pump. (Fig. 3)
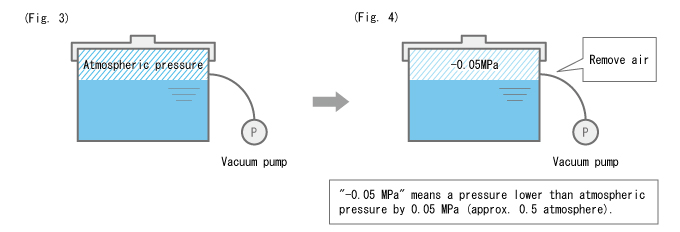
Suppose we lowered the pressure inside the container to -0.05 MPa as shown in Fig. 4. Here, the force pressing down on the liquid surface gets weaker, which makes it easier for water to boil. In other words, the boiling point is lower--water boils at a temperature lower than 100°C. As might be expected, the lower the pressure, the lower the boiling point will also be.
Specifically, water boils at about 80°C under -0.05 MPa, at about 60°C under -0.08 MPa, and at around 45°C under -0.09 MPa.
Remember the working principle of the diaphragm pump?
When the diaphragm of the diaphragm pump moves out, negative pressure develops inside the pump head.
The same effect as shown in Fig. 4 is occurring inside the pump head of a diaphragm pump.
For example, if warm water of 60°C is transferred by a diaphragm pump, when a pressure drop of about 0.08 MPa occurs inside the pump head or in the suction-side piping, this water will boil.
Furthermore, water boiling inside the pump means that gas enters into the pump head, which significantly reduces the efficiency of the diaphragm pump.
Like we have observed here, liquid turning into gas because of a pressure drop (negative pressure) inside the pump head or in the suction-side piping is called cavitation.
We have already discussed in detail how inertial resistance occurs from pulsation of a diaphragm pump in "2-3. Pulsation: Inertial Resistance." The inertial resistance on the discharge side creates increased pressure, but inertial resistance on the suction side creates a drop in pressure. Therefore, when the inertial resistance on the suction side gets excessive, the pressure drop becomes significant, which results in a lower boiling point, allowing for easier vaporization. This is cavitation.
In summary, the factors that facilitate cavitation are, as in the case of inertial resistance:
- a)
Occurs more easily with longer suction piping (hose).
- b)
Occurs more easily with narrower suction piping (hose).
- c)
Occurs more easily with faster stroke speed.
- d)
Occurs more easily with higher pump suction height. (Fig. 1)
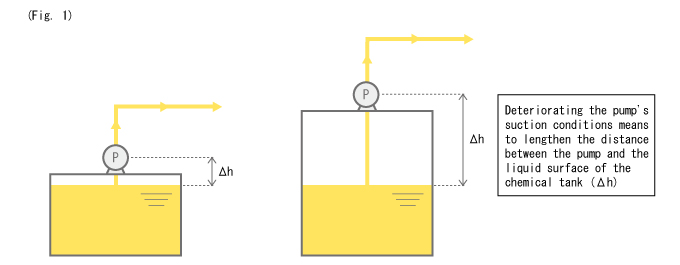
The same can be said about the suction side of a standard d&iaphragm pump or a dual Smoothflow Pump configuration. However, a dual Smoothflow Pump configuration has a faster suction speed, which implies that the inertial resistance from pulsation is greater than that of a standard diaphragm pump, provided that the discharge volume is the same. In other words, cavitation will likely occur if the piping design is inadequate in a dual Smoothflow Pump configuration.
There are also other factors that can foster cavitation, aside from a) through d) above. These factors are related to the nature of the liquid.
- e)
Occurs more easily with higher liquid temperature.
- f)
Occurs more easily with liquids that vaporize easily (i.e. volatile liquids or liquids with low boiling points). Examples include, methanol, acetone, and other low-molecular-weight organic solvents.