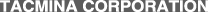Basics|Precision pumping technology 7-1. Cavitation
In a previous section, we reviewed the issues associated with the pulsation of a diaphragm pump ("2-3. Pulsation: Inertial Resistance" and "3-1. A Common Problem Caused by Overfeeding"). Here, we will explain the other important problem: cavitation.
The term "cavitation" was originally used in relation to rotary pumps, such as centrifugal pumps.
While the mechanism in the example we use is different from the mechanism in pumps, it essentially works in the same way and will be useful to help you understand cavitation. Our example is beer.
Let's take a well-chilled bottled beer that has just been taken out of the fridge. Of course, the cap is still on. You see no bubbles in the beer inside the bottle.
However, once you take the cap off, the beer starts bubbling.
If the temperature is high or if you shook the bottle beforehand, even more bubbles form and diffuse out. Why does this happen?
Remember what happens in an air chamber? We have reviewed that more air dissolves into liquid at higher pressures. (assuming the temperature remains constant)
In the case of beer, basically the same applies, with just the beer bottle in place of the air chamber, and carbon dioxide gas in place of air.
As such, an unopened bottle of beer has a few atmospheres of carbon dioxide dissolved in it. The moment it is opened, the pressure drops to atmospheric pressure, and the extra carbon dioxide gas comes out as bubbles.
To put it another way, the difference between the amount of carbon dioxide that can dissolve into beer under a few atmospheres and that under 1 atmosphere can no longer stay dissolved in the beer and becomes bubbles.
When left in this state, the bubbles gradually subside and finally disappear. This means that the amount of dissolved carbon dioxide has reached equilibrium with the atmospheric pressure. By no means the beer has lost all its carbonation.
Let's think of a case of transferring beer with its carbonation in equilibrium with atmospheric pressure (that is, flat beer) using a diaphragm pump.
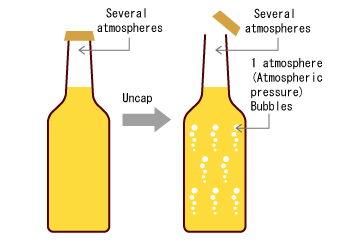
The beer in the tank is flat, that is to say, it has an amount of carbon dioxide dissolved in it that is in balance with atmospheric pressure. In this example, let's assume that the pump is installed on top of the tank. Fig. 1 (1)
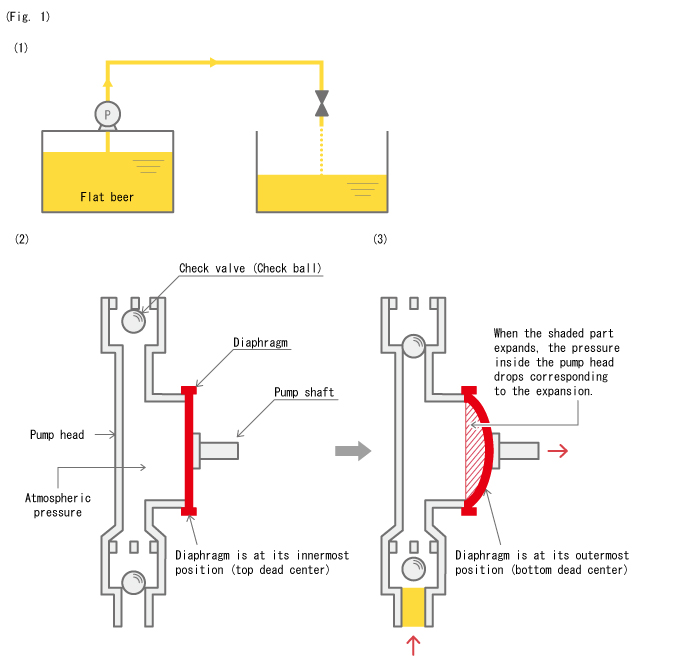
Now looking at the pump in Fig. 1 (2), the pressure inside the pump head is in equilibrium with atmospheric pressure, and no carbon dioxide gas is released from the beer.
Next, what will happen when the diaphragm moves outward as in Fig. 1 (3) and the pressure inside the pump head drops? (See Boyle's law in "4-1. Air Chamber")
Yes, the beer starts bubbling again.
This time, the extra carbon dioxide that cannot dissolve in the beer after saturation (i.e. liquid reaches the point where no more gas can dissolve under a certain pressure) at the dropped pressure is released. In this state, not only beer, but the carbon dioxide gas in the form of bubbles also enters the pump head, which drastically deteriorates the pump's performance. Increased resistance in the piping at the suction side also facilitates gas production.
The "Tank-Over-Pump" Configuration Works for Such Cases
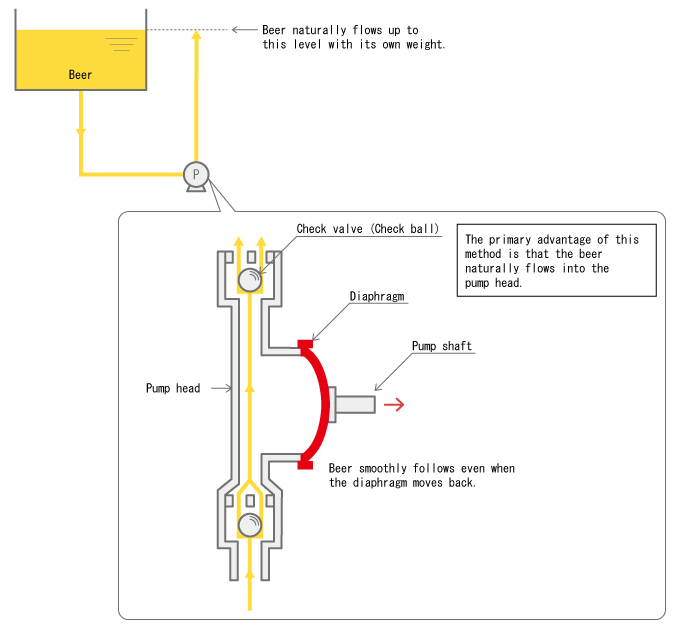
Therefore, even when the diaphragm moves outward, beer smoothly enters the pump head without producing any gas.
However, this method is only effective when the pressure loss in the suction-side piping is small. To explain in detail, if the force of the flow of beer from a higher place to a lower place is greater than the sum of the drop in the pressure inside the pump head and the pressure loss in the suction-side piping, no bubbles will be generated from the flat beer.
This method is not limited to beer, but is also useful for any other liquid that easily bubbles, for example, carbonated water and sodium hypochlorite.